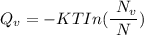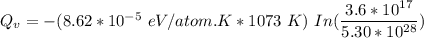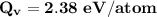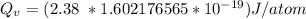Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2



Chemistry, 23.06.2019 21:00
Sulfuryl dichloride may be formed from the reaction of sulfur dioxide and chlorine. so2(g) + cl2(g) → so2cl2(g) substance: so2(g) cl2(g) so2cl2(g) δh°f (kj/mol) at 298 k –296.8 0 –364.0 δg°f (kj/mol) at 298 k –300.1 0 –320.0 s°(j/k • mol) at 298 k 248.2 223.0 311.9 what is δg°rxn for this reaction at 600 k?
Answers: 2
You know the right answer?
Calculate the energy (in J/atom) for vacancy formation in silver, given that the equilibrium number...
Questions

Mathematics, 06.04.2021 06:40

Mathematics, 06.04.2021 06:40

Physics, 06.04.2021 06:40


Biology, 06.04.2021 06:40


Mathematics, 06.04.2021 06:40







Mathematics, 06.04.2021 06:40

Social Studies, 06.04.2021 06:40

Physics, 06.04.2021 06:40

Physics, 06.04.2021 06:40

English, 06.04.2021 06:40

Mathematics, 06.04.2021 06:40

Spanish, 06.04.2021 06:40

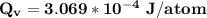
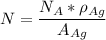
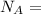 avogadro's number =
avogadro's number = 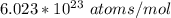
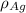 = Density of silver = 9.5 g/cm³
= Density of silver = 9.5 g/cm³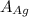 = Atomic weight of sliver = 107.9 g/mol
= Atomic weight of sliver = 107.9 g/mol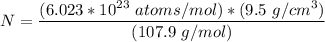
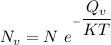
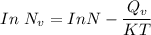
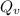 the subject of the formula; we have:
the subject of the formula; we have: