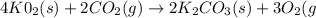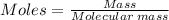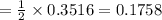Chemistry, 02.07.2020 15:01 MalikaJones
A self-contained breathing apparatus can be a life-saving piece of personal protective equipment (PPE) for first responders. SCBA provide breathing oxygen in a contained system for use in low-oxygen environments or in the presence of toxic fumes. The oxygen is generated through the reaction of potassium superoxide, KO2 and carbon dioxide, forming potassium carbonate and oxygen gas. If the 25.0 g KO2 in the SCBA system was exposed to 45.0 g CO2, what scenario best describes the outcome:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Silica, sio2, is formed on silicon as an electrically insulating layer for microelectronic devices. silica is formed when silicon is exposed to o2 gas at an elevated temperature. at 900˚c, it takes 90 minutes for the oxygen to diffuse from the surface to form a 0.06 micron (0.06 x 10-6 m) thick layer of sio2 on
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 23.06.2019 13:00
The gram molecular mass or co2 is the same as the gram molecular mass of
Answers: 2

Chemistry, 23.06.2019 13:30
Asap what is the temperature when the volume is 700 ml? a 500 k b 200 k c 600 k d 700 k
Answers: 1
You know the right answer?
A self-contained breathing apparatus can be a life-saving piece of personal protective equipment (PP...
Questions


Mathematics, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00


Geography, 27.02.2021 14:00



Biology, 27.02.2021 14:00

Law, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00




Spanish, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00


World Languages, 27.02.2021 14:00

Biology, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00