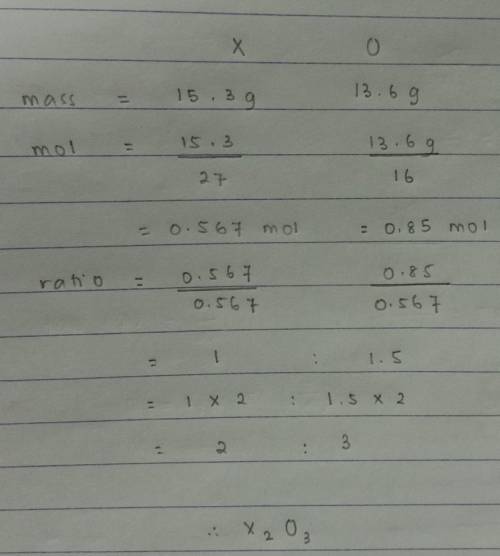Chemistry, 27.06.2020 15:01 Tariah5970
Quick answer plzAn oxide was prepared by combining 15.3g of an element “X” with 13.6g of oxygen. What is the simplest formula for the oxide? (At. Mass X = 27, O = 16)
A. XO3
B. X2O3
C. X2O5
D. XO

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
Quick answer plzAn oxide was prepared by combining 15.3g of an element “X” with 13.6g of oxygen. Wha...
Questions


Mathematics, 06.10.2019 13:30

English, 06.10.2019 13:30

History, 06.10.2019 13:30


Mathematics, 06.10.2019 13:30





Computers and Technology, 06.10.2019 13:30



History, 06.10.2019 13:30




English, 06.10.2019 13:30

Mathematics, 06.10.2019 13:30

Computers and Technology, 06.10.2019 13:30