1do the following diagrams represent isotopes of the same elements? explain.
2 what are the m...

Chemistry, 06.10.2019 13:30 Fairy1108789
1do the following diagrams represent isotopes of the same elements? explain.
2 what are the mass numbers of these atoms? explain ur reasoning.
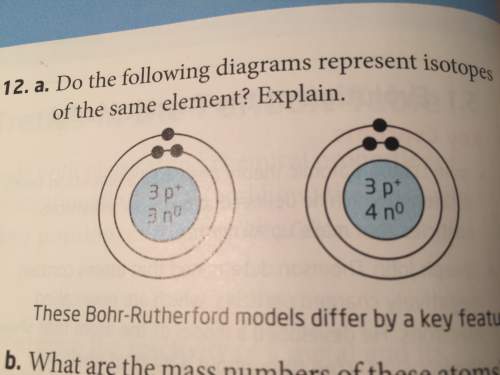

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Questions

Mathematics, 13.02.2021 07:00

World Languages, 13.02.2021 07:00



History, 13.02.2021 07:00

History, 13.02.2021 07:00

Mathematics, 13.02.2021 07:00

Mathematics, 13.02.2021 07:00

Mathematics, 13.02.2021 07:00

Advanced Placement (AP), 13.02.2021 07:00



History, 13.02.2021 07:00




Biology, 13.02.2021 07:00

History, 13.02.2021 07:00

Mathematics, 13.02.2021 07:00



