
Chemistry, 21.06.2020 03:57 rileyeddins1010
4. Set-up ONLY the equation for determining the bond dissociation energy ΔH° for the hydrogenation of acetylene, C2H2 in the reaction below by showing what bonds are being broken (yes, draw Lewis Structures!) and what bonds are being formed: H-C=C-H(g) + 2 H2(g) → CH3-CH3(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1

Chemistry, 23.06.2019 10:10
Which orbitals form a pi bond? a. the s orbital and three p orbitals b. the s orbital and two p orbitals c. overlapping p orbitals d. overlapping hybrid orbitals
Answers: 2
You know the right answer?
4. Set-up ONLY the equation for determining the bond dissociation energy ΔH° for the hydrogenation o...
Questions


Biology, 17.06.2021 20:00


English, 17.06.2021 20:00


Mathematics, 17.06.2021 20:00



Geography, 17.06.2021 20:00


Mathematics, 17.06.2021 20:00


Mathematics, 17.06.2021 20:00

Computers and Technology, 17.06.2021 20:00


Mathematics, 17.06.2021 20:00

Biology, 17.06.2021 20:00



![\Delta H=[(1\times B.E_{C\equiv C})+(2\times B.E_{C-H})}+(2\times B.E_{H-H}) ]-[(1\times B.E_{C-C})+(6\times B.E_{C-H})]](/tpl/images/0691/2872/187e8.png)
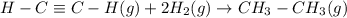
![\Delta H=\sum [n\times B.E(reactant)]-\sum [n\times B.E(product)]](/tpl/images/0691/2872/42942.png)
![\Delta H=[(n_{H-C\equiv C-H}\times B.E_{H-C\equiv C-H})+(n_{H_2}\times B.E_{H_2}) ]-[(n_{CH_3-CH_3}\times B.E_{CH_3-CH_3})]](/tpl/images/0691/2872/fadbe.png)
![\Delta H=[(1\times B.E_{C\equivC})+(2\times B.E_{C-H})}+(2\times B.E_{H-H}) ]-[(1\times B.E_{C-C})+(6\times B.E_{C-H})]](/tpl/images/0691/2872/5ee06.png)


