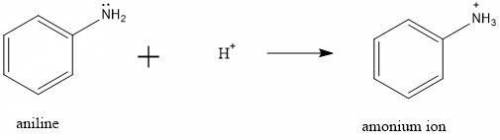Chemistry, 16.06.2020 20:57 rayniqueamee2002
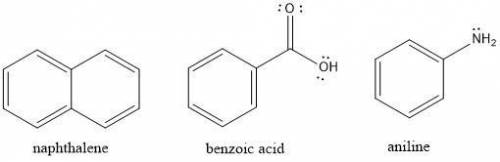
Suppose you have crude reaction mixture containing napthalene, benzoic acid, and aniline dissolved in an organic solvent, and you wish to extract the different molecules by altering the solubility of each component in solution. Which of the following statements would be true?
a. Adding 5% HCl solution to the crude reaction mixture will deprotonate benzoic acid increasing its solubility in the aqueous solution.
b. Adding 5% NaOH solution to the crude reaction mixture will protonate napthalene making it more soluble in the aqueous solution.
c. Adding 5% HCl solution to the crude reaction mixture will protonate napthalene making it more soluble in the aqueous solution.
d. Adding 5% HCl solution to the crude reaction mixture will protonate aniline increasing its solubility in the aqueous solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Suppose you have crude reaction mixture containing napthalene, benzoic acid, and aniline dissolved i...
Questions



Social Studies, 22.04.2020 09:17

SAT, 22.04.2020 09:17



Mathematics, 22.04.2020 09:17

Mathematics, 22.04.2020 09:17


Mathematics, 22.04.2020 09:17

Chemistry, 22.04.2020 09:18



History, 22.04.2020 09:18


Mathematics, 22.04.2020 09:18


Mathematics, 22.04.2020 09:18


Advanced Placement (AP), 22.04.2020 09:18

 the presence of this hydronium ion will protonate the acid, so we can discard a.
the presence of this hydronium ion will protonate the acid, so we can discard a.