
Chemistry, 13.06.2020 09:57 hmskevinjacobo5471
I NEED HELP PLEASE, THANKS!
Ammonia (NH3) is an example of a Brønsted-Lowry Base.
-Define the Brønsted-Lowry acid-base theory.
-What is the pH of an ammonia solution that has a concentration of 0.335 M? The Kb of ammonia is 1.8 × 10^–5.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make these assumptions: the combined weight of the pilot basket together with that of the balloon fabric and other equipment is w. the volume of the hot air inside the balloon when it is inflated is v. the absolute temperature of the hot air at the bottom of the balloon is th (where th> tc). the absolute temperature of the cold air outside the balloon is tc and its density is ďc. the balloon is open at the bottom, so that the pressure inside and outside the balloon is the same. as always, treat air as an ideal gas. use g for the magnitude of the acceleration due to gravity.
Answers: 1

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2


Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1
You know the right answer?
I NEED HELP PLEASE, THANKS!
Ammonia (NH3) is an example of a Brønsted-Lowry Base.
-Define the...
-Define the...
Questions

Mathematics, 24.07.2020 06:01

English, 24.07.2020 06:01

Mathematics, 24.07.2020 06:01

History, 24.07.2020 06:01

History, 24.07.2020 06:01

English, 24.07.2020 06:01


Biology, 24.07.2020 06:01


Mathematics, 24.07.2020 06:01

Mathematics, 24.07.2020 06:01



Advanced Placement (AP), 24.07.2020 06:01

Mathematics, 24.07.2020 06:01

Mathematics, 24.07.2020 06:01

Mathematics, 24.07.2020 06:01


Mathematics, 24.07.2020 06:01

Mathematics, 24.07.2020 06:01

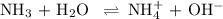
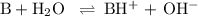
![\rm K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.335 - x} = 1.8 \times 10^{-5}](/tpl/images/0685/0322/975fe.png)
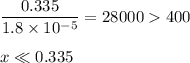
![\dfrac{x^{2}}{0.335} = 1.8 \times 10^{-5}\\\\x^{2} = 0.335 \times 1.8 \times 10^{-5}\\x^{2} = 6.03 \times 10^{-6}\\x = \sqrt{6.03 \times 10^{-6}}\\x = \text{[OH]}^{-} = \mathbf{2.46 \times 10^{-3}} \textbf{ mol/L}](/tpl/images/0685/0322/0aa42.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(2.46 \times 10^{-3}) = 2.61](/tpl/images/0685/0322/acbc3.png)


