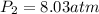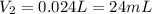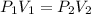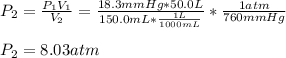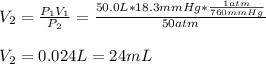Chemistry, 29.05.2020 03:02 Franklyn3834
3. A 50.0 L sample of gas collected in the upper atmosphere at a pressure of 18.3 mmHg is compressed into a 150.0 mL container at the same temperature.
a. What is the new pressure, in atm?
b. To what volume would the original sample have had to be compressed to exert a pressure of 10.0 atm?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

You know the right answer?
3. A 50.0 L sample of gas collected in the upper atmosphere at a pressure of 18.3 mmHg is compressed...
Questions




Mathematics, 02.08.2019 07:10




Mathematics, 02.08.2019 07:10




Mathematics, 02.08.2019 07:10