Chemistry, 23.05.2020 16:59 milkshakegrande101
A geochemist in the field takes a 34.0 mL sample of water from a rock pool lined with crystals of a certain mineral compound X. He notes the temperature of the pool, 22.0 °C, and caps the sample carefully. Back in the lab, the geochemist first dilutes the sample with distilled water to 750.0 mL. Then he filters it and evaporates all the water under vacuum. Crystals of X are left behind. The researcher washes, dries, and weighs the crystals. They weigh 0.31 g.
Using only the information above, can you calculate yes the solubility of X in the water at 17.0 °C? If yes, calculate it. Be sure your answer has a unit symbol and 3 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
A geochemist in the field takes a 34.0 mL sample of water from a rock pool lined with crystals of a...
Questions

English, 20.12.2021 09:30


Mathematics, 20.12.2021 09:30

Chemistry, 20.12.2021 09:30



Mathematics, 20.12.2021 09:30


Physics, 20.12.2021 09:40

English, 20.12.2021 09:40

History, 20.12.2021 09:40

English, 20.12.2021 09:40


Mathematics, 20.12.2021 09:40


Mathematics, 20.12.2021 09:40




Mathematics, 20.12.2021 09:40

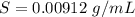
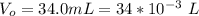
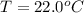
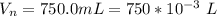
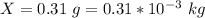
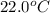 can be mathematically evaluate as
can be mathematically evaluate as