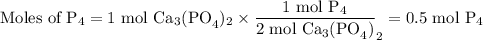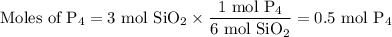Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 11:00
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2

Chemistry, 23.06.2019 20:00
Within nonmetals,ions that have more electrons tend to be bigger than ions that have fewer additional electrons why?
Answers: 1

Chemistry, 23.06.2019 22:30
How did scientists obtain environmental dna (edna) from carp in the waterways near chicago
Answers: 2

Chemistry, 24.06.2019 02:00
A0.1 mm sample of human blood has approximately 6000 red blood cells. an adult typically has 5.0 l of blood. how many red blood cells are in an adult? (1 ml = 1 cc). use "e" for scientific notation.
Answers: 1
You know the right answer?
How many moles of P4 will form when 1 mole of Ca3(PO4)2 reacts with 3 moles of SiO2 and 2 moles of C...
Questions

Mathematics, 25.05.2021 14:00

Mathematics, 25.05.2021 14:00



English, 25.05.2021 14:00

Mathematics, 25.05.2021 14:00

Physics, 25.05.2021 14:00

History, 25.05.2021 14:00

Mathematics, 25.05.2021 14:00



Mathematics, 25.05.2021 14:00

English, 25.05.2021 14:00

Computers and Technology, 25.05.2021 14:00

Physics, 25.05.2021 14:00

Chemistry, 25.05.2021 14:00

Mathematics, 25.05.2021 14:00

Social Studies, 25.05.2021 14:00

Mathematics, 25.05.2021 14:00