How many moles of H2O will be formed from the reaction of 80 g of
NaOH? *
H2SO4 + 2 NaOH...

Chemistry, 05.05.2020 03:43 mimireds8573
How many moles of H2O will be formed from the reaction of 80 g of
NaOH? *
H2SO4 + 2 NaOH = Na2SO4 + 2 H 0
0.5 moles
1 mole
2 moles
4 moles

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1

Chemistry, 23.06.2019 10:00
Compare and contrast an assemblage and a pollen fingerprint by defying both and giving examples of each from the chapter.
Answers: 3

You know the right answer?
Questions

Mathematics, 20.01.2020 18:31




Mathematics, 20.01.2020 18:31















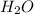 will be formed.
will be formed.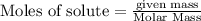
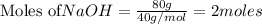
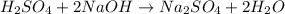
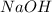 give = 2 moles of
give = 2 moles of 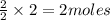 of
of 


