

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Determine the number of moles of oxygen atoms in each of the following: 4.49 mol h2o2, 2.17 mol n2o...
Questions

Computers and Technology, 30.07.2019 19:00




English, 30.07.2019 19:00

Mathematics, 30.07.2019 19:00

Biology, 30.07.2019 19:00

Chemistry, 30.07.2019 19:00

History, 30.07.2019 19:00


English, 30.07.2019 19:00

Mathematics, 30.07.2019 19:00


Biology, 30.07.2019 19:00


Mathematics, 30.07.2019 19:00

Mathematics, 30.07.2019 19:00



Health, 30.07.2019 19:00

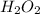 contains 8.98 moles of oxygen atoms.
contains 8.98 moles of oxygen atoms. 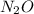 contains 2.17 moles of oxygen atoms.
contains 2.17 moles of oxygen atoms.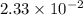 mol
mol 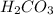 contains 0.0699 moles of oxygen atoms.
contains 0.0699 moles of oxygen atoms.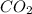 contains 48.6 moles of oxygen atoms.
contains 48.6 moles of oxygen atoms.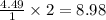 moles of oxygen atoms
moles of oxygen atoms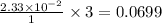 moles of oxygen atoms
moles of oxygen atoms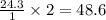 moles of oxygen atoms
moles of oxygen atoms

