
Chemistry, 03.05.2020 13:04 Kaziyah461
0.80 mol MgBr2 is added to 1.00 kg water. Determine the freezing point of the solution. Water has a
freezing point depression constant of 1.86°C. kg/mol.
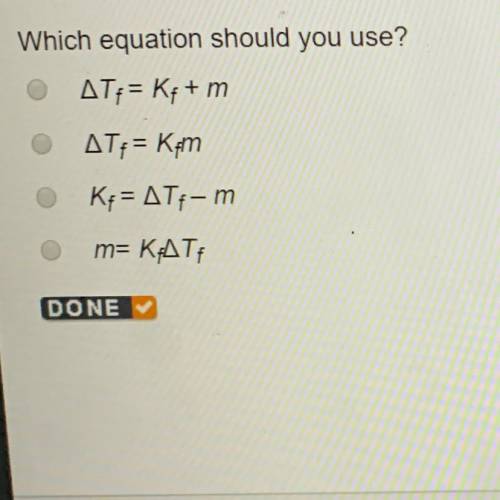
Which equation should you use?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
0.80 mol MgBr2 is added to 1.00 kg water. Determine the freezing point of the solution. Water has a<...
Questions

English, 12.10.2019 15:50



Biology, 12.10.2019 15:50


Mathematics, 12.10.2019 15:50




Geography, 12.10.2019 15:50

Mathematics, 12.10.2019 15:50











