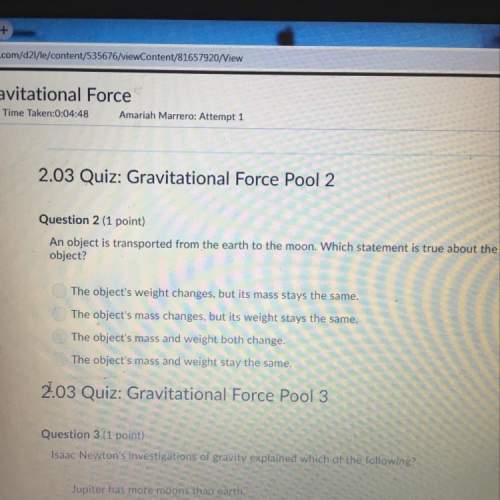
Chemistry, 05.05.2020 01:14 thompsonhomes1
4FeCr2O7+ 8K2CO3 + 1 O22Fe2O3+ 8K2CrO4 + 8CO2
(a) How many molesof FeCr2O7are required to produce 44 molesof CO2?
(b) How many moles of O2are required to produce 107.9 molesof Fe2O3?
(c) If 309 molesof FeCr2O7react, how many molesof O2will be consumed?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
4FeCr2O7+ 8K2CO3 + 1 O22Fe2O3+ 8K2CrO4 + 8CO2
(a) How many molesof FeCr2O7are required t...
(a) How many molesof FeCr2O7are required t...
Questions