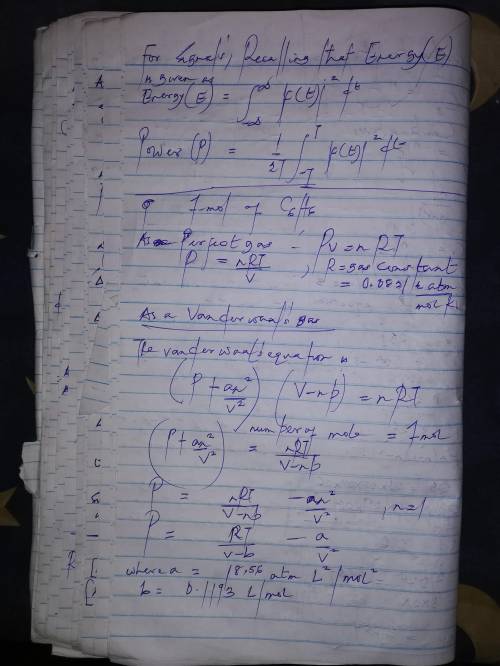Chemistry, 28.01.2020 02:31 Desinfektionsmittel
Calculate the pressure exerted by 1.0 mol of c6h6behaving as a)a perfect gas and b) a van der waals gas when it is confined under the following conditions: i)373.15 k in 22.414 dm3ii)1000 k in 22.414 dm3iii)1000 k in 150.000 dm3at which one of these conditions does the real gas be?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3
You know the right answer?
Calculate the pressure exerted by 1.0 mol of c6h6behaving as a)a perfect gas and b) a van der waals...
Questions

History, 08.10.2021 07:20

Social Studies, 08.10.2021 07:20




Social Studies, 08.10.2021 07:20

Social Studies, 08.10.2021 07:20


Arts, 08.10.2021 07:20

Mathematics, 08.10.2021 07:20



Chemistry, 08.10.2021 07:20


Chemistry, 08.10.2021 07:20

Business, 08.10.2021 07:20

Mathematics, 08.10.2021 07:20

English, 08.10.2021 07:20

Social Studies, 08.10.2021 07:20