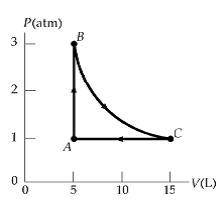
II. Practice An ideal gas occupies 5 L at atmospheric pressure and 300 K (point A). It is warmed at constant volume to 3 atm (point B). Then it is allowed to expand isothermally to 1 atm (point C) and at last compressed isobarically to its original state. A. How many moles of gas are being used? B. Find the temperature at point C. C. Find the work done on the gas in each process. D. Find the amount of heat added to/removed from the gas in one cycle.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

You know the right answer?
II. Practice An ideal gas occupies 5 L at atmospheric pressure and 300 K (point A). It is warmed at...
Questions

Physics, 11.01.2022 15:40

Biology, 11.01.2022 15:40


Mathematics, 11.01.2022 15:50

English, 11.01.2022 15:50

Chemistry, 11.01.2022 15:50

Mathematics, 11.01.2022 15:50

Health, 11.01.2022 15:50


Mathematics, 11.01.2022 15:50


Health, 11.01.2022 15:50


Mathematics, 11.01.2022 15:50

History, 11.01.2022 15:50


Mathematics, 11.01.2022 16:00


History, 11.01.2022 16:00