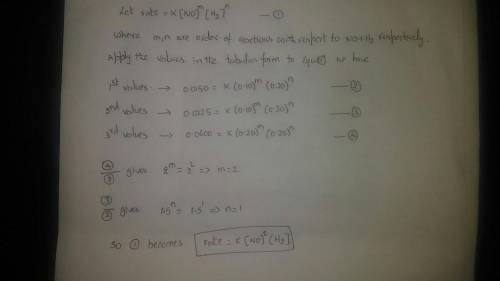Rate data have been determined at a particular temperature for the overall reaction 2NO(g) + 2H2(g) → N2(g) + 2H2O(g). The value of the specific rate constant at this temperature is ___.
Trial Run Initial [NO] Initial [H2] Initial Rate (M•s–1)
1 0.10 M 0.20 M 0.0150
2 0.10 M 0.30 M 0.0225
3 0.20 M 0.20 M 0.0600

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

You know the right answer?
Rate data have been determined at a particular temperature for the overall reaction 2NO(g) + 2H2(g)...
Questions




Social Studies, 01.07.2019 22:00

Mathematics, 01.07.2019 22:00



Chemistry, 01.07.2019 22:00




Mathematics, 01.07.2019 22:00


Social Studies, 01.07.2019 22:00


English, 01.07.2019 22:00




Mathematics, 01.07.2019 22:00