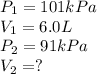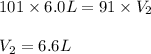Chemistry, 25.04.2020 01:40 deaishaajennings123
In one city, a balloon with a volume of 6.0 L is filled with air at 101 kPa
pressure. The balloon is then taken to a second city at a much higher
altitude. At this second city, atmospheric pressure is only 91 kPa. If the
temperature is the same in both places, what will be the new volume of the
balloon?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1
You know the right answer?
In one city, a balloon with a volume of 6.0 L is filled with air at 101 kPa
pressure. The ball...
pressure. The ball...
Questions





Mathematics, 07.01.2021 21:40

Mathematics, 07.01.2021 21:40




Mathematics, 07.01.2021 21:40


History, 07.01.2021 21:40


Mathematics, 07.01.2021 21:40

Mathematics, 07.01.2021 21:40


SAT, 07.01.2021 21:40



English, 07.01.2021 21:40

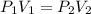
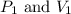 are initial pressure and volume.
are initial pressure and volume.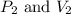 are final pressure and volume.
are final pressure and volume.