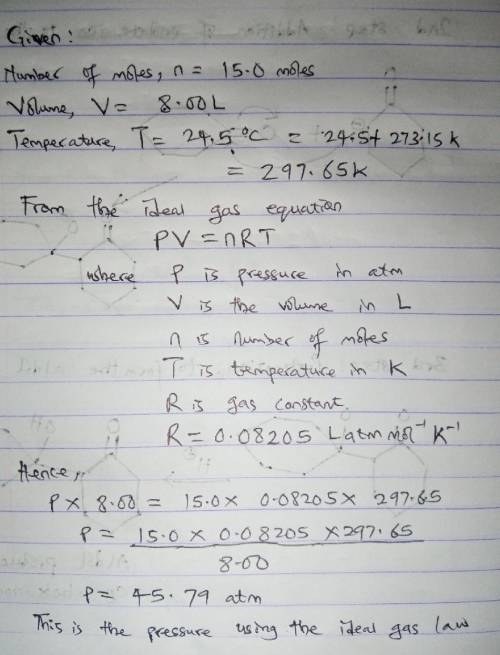Chemistry, 22.04.2020 02:08 awesomegrill
15.0 moles of gas are in a 8.00 LL tank at 24.5 ∘C∘C . Calculate the difference in pressure between methane and an ideal gas under these conditions. The van der Waals constants for methane are a=2.300L2⋅atm/mol2a=2.300L2⋅atm/mol 2 and b=0.0430 L/molb=0.0430 L/mol .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 11:00
Afraction can be converted to a decimal by dividing the denominator into the numerator. use a calculator. divide to convert the fractions from part a to decimals. then describe the pattern you see in the decimal.
Answers: 3
You know the right answer?
15.0 moles of gas are in a 8.00 LL tank at 24.5 ∘C∘C . Calculate the difference in pressure between...
Questions


Mathematics, 12.02.2021 06:40

Mathematics, 12.02.2021 06:40



History, 12.02.2021 06:40


Mathematics, 12.02.2021 06:40


History, 12.02.2021 06:40

History, 12.02.2021 06:40

Biology, 12.02.2021 06:40



Mathematics, 12.02.2021 06:40

Mathematics, 12.02.2021 06:40

Mathematics, 12.02.2021 06:40

English, 12.02.2021 06:40