
Chemistry, 21.04.2020 07:00 savannahworkman11
COLLEGE CHEMISTRY 35 POINTS
Determine the percent yield when 12 g of CO2 are formed experimentally from the reaction of 0.5 moles of C8H18 reacting with excess oxygen according to the following balanced equation:
2 C8H18(l) + 25 O2(g) 16 CO2(g) + 18 H2O(l)
Please show your work.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
COLLEGE CHEMISTRY 35 POINTS
Determine the percent yield when 12 g of CO2 are formed experiment...
Determine the percent yield when 12 g of CO2 are formed experiment...
Questions

Mathematics, 22.02.2021 19:00

Mathematics, 22.02.2021 19:00





Computers and Technology, 22.02.2021 19:00





Computers and Technology, 22.02.2021 19:00

English, 22.02.2021 19:00



Computers and Technology, 22.02.2021 19:00




Physics, 22.02.2021 19:00

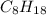 = 0.5
= 0.5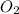 ⇒ 16 C
⇒ 16 C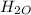
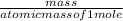
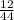
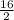 =
= 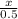
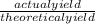 x 100
x 100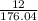 x 100
x 100

