
A 9.75 L 9.75 L container holds a mixture of two gases at 41 ° C. 41 °C. The partial pressures of gas A and gas B, respectively, are 0.419 atm 0.419 atm and 0.589 atm. 0.589 atm. If 0.240 mol 0.240 mol of a third gas is added with no change in volume or temperature, what will the total pressure become?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
A 9.75 L 9.75 L container holds a mixture of two gases at 41 ° C. 41 °C. The partial pressures of ga...
Questions


History, 01.03.2021 07:30




Mathematics, 01.03.2021 07:30

History, 01.03.2021 07:30




Mathematics, 01.03.2021 07:30

Mathematics, 01.03.2021 07:30








Mathematics, 01.03.2021 07:30

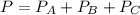 ----- (1)
----- (1)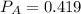 atm
atm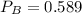 atm
atm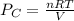
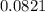
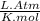
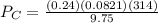
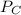 = 0.634 atm
= 0.634 atm

