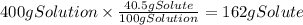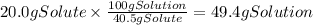Chemistry, 14.04.2020 22:30 hannahblank2466
A chemistry student needs of acetic acid for an experiment. He has available of a w/w solution of acetic acid in acetone. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 07:20
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3
You know the right answer?
A chemistry student needs of acetic acid for an experiment. He has available of a w/w solution of ac...
Questions


Biology, 15.12.2021 04:30

Mathematics, 15.12.2021 04:30

Computers and Technology, 15.12.2021 04:30

Chemistry, 15.12.2021 04:30

History, 15.12.2021 04:30

Mathematics, 15.12.2021 04:30


History, 15.12.2021 04:30



Social Studies, 15.12.2021 04:30

Computers and Technology, 15.12.2021 04:30


Mathematics, 15.12.2021 04:30

Chemistry, 15.12.2021 04:30

Health, 15.12.2021 04:30

Physics, 15.12.2021 04:30

English, 15.12.2021 04:30

English, 15.12.2021 04:30