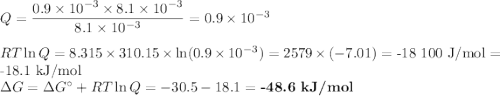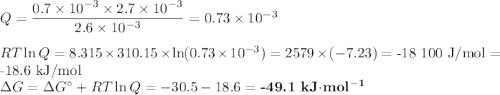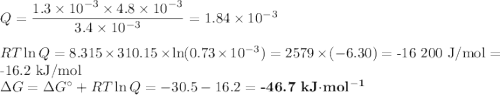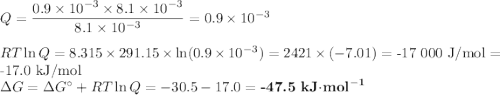Chemistry, 11.04.2020 01:50 ariellllllllllllllll
The equation for ATP hydrolysis is
ATP yields ADP + Pi delta G*= -30.5 kJ/mol
( H20)
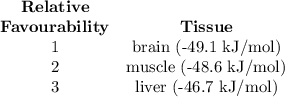
(a) Calculate ?G for ATP hydrolysis to rank the following conditions from most favorable to least favorable. Assume a temperature of 37.0C. R = 8.315 J/(mol. K).
ATP Hydrolysis most favorable
ATP hydroysis least favorable
The choices are:
a. muscle: [ATP]= 8.1mM; [ADP]= 0.9mM [Pi]= 8.1mM
b. brain: [ATP]= 2.6mM; [ADP]= 0.7mM [Pi]= 2.7mM
c. liver: [ATP]= 3.4mM; [ADP]= 1.3mM [Pi]= 4.8mM
(b) Calculate ?G for ATP hydrolysis in muscle at 18 degree C. Use the muscle concentrations from part a.
Delta g = kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3
You know the right answer?
The equation for ATP hydrolysis is
ATP yields ADP + Pi delta G*= -30.5 kJ/mol
( H2...
ATP yields ADP + Pi delta G*= -30.5 kJ/mol
( H2...
Questions











Computers and Technology, 12.08.2020 06:01



Business, 12.08.2020 06:01


History, 12.08.2020 06:01


Social Studies, 12.08.2020 06:01

Computers and Technology, 12.08.2020 06:01

![Q = \dfrac{\text{[ADP][P$_{\text{i}}$]}}{\text{[ADP]}}](/tpl/images/0594/8302/09bb9.png)