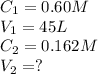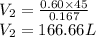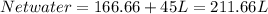In some regions of the southwest United States, the water is very hard. For example, in Las Cruces, New Mexico, the tap water contains about 560 mg of dissolved solids per milliliter. Reverse osmosis units are marketed in this area to soften water. A typical unit exerts a pressure of 8.0 atm and can produce 45 L water per day
a. Assuming all of the dissolved solids are Mgco3 and assuming a temperature of 27 degrees, what total volume of water must be processed to produce 45 L pure water?
b. Would the same system work for purifying seawater? (Assume seawater is 0.60 MNaCl)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 02:30
At 40 âc the solution has at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.g of kno3 per 100 g of water and it can contain up to at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.g of kno3 per 100 g of water. at 0 âc the solubility is ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.kno3 per 100 g of water, so ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.gkno3 per 100 g of water will precipitate out of solution. a kno3 solution containing 55 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 2

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

You know the right answer?
In some regions of the southwest United States, the water is very hard. For example, in Las Cruces,...
Questions




Computers and Technology, 28.12.2019 05:31





Computers and Technology, 28.12.2019 05:31











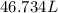 of hard water is require to produce
of hard water is require to produce 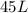 of pure water.
of pure water.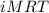
 is van't hoff factor
is van't hoff factor 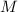 is molar concentration (mol/L)
is molar concentration (mol/L)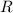 is gas constant
is gas constant 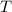 is Temperature in Kelvin
is Temperature in Kelvin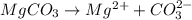
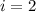
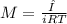
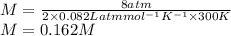
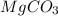
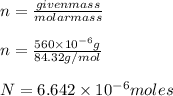
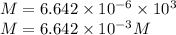
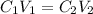
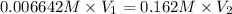
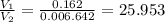
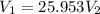
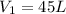
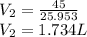
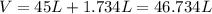
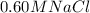 as well,
as well,