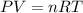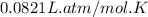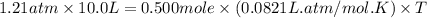Chemistry, 07.04.2020 19:12 keshan3000
Oxygen gas is collected at a pressure of 1.21 atm in a container which has a volume of 10.0 L. What temperature (in Kelvin) must be maintained on 0.500 moles of this gas in order to maintain this pressure? Round to the nearest whole number (no decimals in your answer).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1
You know the right answer?
Oxygen gas is collected at a pressure of 1.21 atm in a container which has a volume of 10.0 L. What...
Questions


Mathematics, 30.01.2020 12:01

History, 30.01.2020 12:01



Mathematics, 30.01.2020 12:01

Chemistry, 30.01.2020 12:01




Physics, 30.01.2020 12:01



Computers and Technology, 30.01.2020 12:01


Mathematics, 30.01.2020 12:01

Biology, 30.01.2020 12:01



Mathematics, 30.01.2020 12:01