Chemistry, 06.04.2020 19:08 cassanovaanthony
Suppose 1.73g of barium nitrate is dissolved in 100.mL of a 63.0mM aqueous solution of sodium chromate.
Calculate the final molarity of nitrate anion in the solution. You can assume the volume of the solution doesn't change when the barium nitrate is dissolved in it. Be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1


You know the right answer?
Suppose 1.73g of barium nitrate is dissolved in 100.mL of a 63.0mM aqueous solution of sodium chroma...
Questions

Computers and Technology, 23.09.2019 18:00

Business, 23.09.2019 18:00

English, 23.09.2019 18:00

Mathematics, 23.09.2019 18:00



History, 23.09.2019 18:00



Mathematics, 23.09.2019 18:00


Social Studies, 23.09.2019 18:00




Physics, 23.09.2019 18:00

Social Studies, 23.09.2019 18:00



Biology, 23.09.2019 18:00

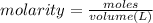
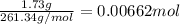
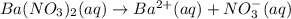
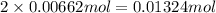 of nitrate ions
of nitrate ions