
Chemistry, 06.04.2020 18:02 wilkoASK2919
For the reaction 2A(g) â B(g), the equilibrium constant is Kp = 0.76. A reaction mixture initially contains 4.0 atm of gas (PA = 2.0 atm and PB = 2.0 atm).
Which statement is true of the reaction mixture?
(a) The reaction mixture will proceed toward products.
(b) The reaction mixture is at equilibrium.
(c) The reaction mixture will proceed toward reactants
(d) It is not possible to determine from the information given the future direction of the reaction mixture

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
For the reaction 2A(g) â B(g), the equilibrium constant is Kp = 0.76. A reaction mixture initially c...
Questions

Mathematics, 29.02.2020 03:28
















Health, 29.02.2020 03:28



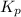

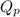 is written as:
is written as:
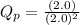
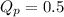

 , the reaction will shift towards the right i.e. towards the product side.
, the reaction will shift towards the right i.e. towards the product side.

