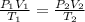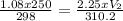Chemistry, 02.04.2020 06:27 zitterkoph
A sample of neon gas at a pressure of 1.08 atm fills a flask with a volume of 250 mL at a temperature of 24.0 °C. If the gas is transferred to another flask at 37.2 °C and a pressure of 2.25 atm, what is the volume of the new flask?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1


Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
A sample of neon gas at a pressure of 1.08 atm fills a flask with a volume of 250 mL at a temperatur...
Questions




Mathematics, 03.04.2020 03:46

Geography, 03.04.2020 03:46

Mathematics, 03.04.2020 03:46

Mathematics, 03.04.2020 03:46

Mathematics, 03.04.2020 03:46

Physics, 03.04.2020 03:46








Mathematics, 03.04.2020 03:46


Mathematics, 03.04.2020 03:47