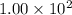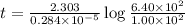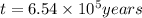Radioactive plutonium−239 (t1/2 = 2.44 × 105 yr) is used in nuclear reactors and atomic bombs. If there are 6.40 × 102 g of the isotope in a small atomic bomb, how long will it take for the substance to decay to 1.00 × 102 g, too small an amount for an effective bomb? (Hint: Radioactive decays follow first-order kinetics.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Radioactive plutonium−239 (t1/2 = 2.44 × 105 yr) is used in nuclear reactors and atomic bombs. If th...
Questions


Mathematics, 19.01.2021 20:20


Mathematics, 19.01.2021 20:20

English, 19.01.2021 20:20


Mathematics, 19.01.2021 20:20

English, 19.01.2021 20:20

Health, 19.01.2021 20:20



Mathematics, 19.01.2021 20:20


Mathematics, 19.01.2021 20:20



Mathematics, 19.01.2021 20:20

Physics, 19.01.2021 20:20


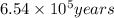
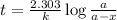
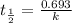
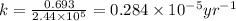
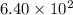 g of the isotope to decay to
g of the isotope to decay to