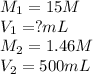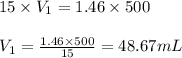Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 18:00
Sylvanite is a mineral that contains 28.0% gold by mass. how much sylvanite would you need to dig up to obtain 77.0 g of gold? explain how you got your answer and the steps you took. you
Answers: 3

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

You know the right answer?
The ammonia solution that is purchased for a stockroom has a molarity of 15 M. Determine the volume...
Questions







Mathematics, 03.07.2021 04:30



Advanced Placement (AP), 03.07.2021 04:30




Mathematics, 03.07.2021 04:30






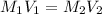
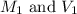 are the molarity and volume of the stock ammonia solution
are the molarity and volume of the stock ammonia solution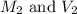 are the molarity and volume of diluted ammonia solution
are the molarity and volume of diluted ammonia solution