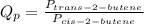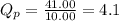The equilibrium constant, Kp, equals 3.40 at 25 ∘C for the isomerization reaction: cis-2-butene ⇋ trans-2-butene. If a flask initially contains 41.00 atm of trans-2-butene and 10.00 atm of cis-2-butene, in what direction will the system shift to reach equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
You know the right answer?
The equilibrium constant, Kp, equals 3.40 at 25 ∘C for the isomerization reaction: cis-2-butene ⇋ tr...
Questions


History, 29.05.2020 03:02












Mathematics, 29.05.2020 03:02

World Languages, 29.05.2020 03:02

Mathematics, 29.05.2020 03:02

Mathematics, 29.05.2020 03:02


Mathematics, 29.05.2020 03:02