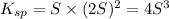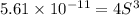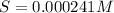Chemistry, 26.03.2020 23:46 tinasidell1972
Mg(OH)2 is a sparingly soluble compound, in this case a base, with a solubility product, Ksp, of 5.61×10−11. It is used to control the pH and provide nutrients in the biological (microbial) treatment of municipal wastewater streams. Based on the given value of the Ksp, what is the molar solubility of Mg(OH)2 in pure H2O?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
You know the right answer?
Mg(OH)2 is a sparingly soluble compound, in this case a base, with a solubility product, Ksp, of 5.6...
Questions

Geography, 06.04.2020 23:46

SAT, 06.04.2020 23:46

Mathematics, 06.04.2020 23:46

History, 06.04.2020 23:46

Mathematics, 06.04.2020 23:46


History, 06.04.2020 23:47

Mathematics, 06.04.2020 23:47



Mathematics, 06.04.2020 23:47

Mathematics, 06.04.2020 23:47

Mathematics, 06.04.2020 23:47


Computers and Technology, 06.04.2020 23:47

Mathematics, 06.04.2020 23:48

Mathematics, 06.04.2020 23:48


Chemistry, 06.04.2020 23:48

Physics, 06.04.2020 23:48

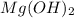 in pure water.
in pure water.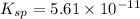
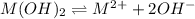
![K_{sp}=[M^{2+}][OH^-]^2](/tpl/images/0566/2963/a461b.png)