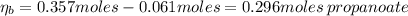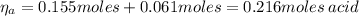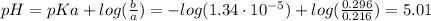A 1.41 L buffer solution consists of 0.253 M propanoic acid and 0.110 M sodium propanoate. Calculate the pH of the solution following the addition of 0.061 mol HCl . Assume that any contribution of the HCl to the volume of the solution is negligible. The K a of propanoic acid is 1.34 × 10 − 5 .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1
You know the right answer?
A 1.41 L buffer solution consists of 0.253 M propanoic acid and 0.110 M sodium propanoate. Calculate...
Questions

English, 22.06.2019 02:00







Mathematics, 22.06.2019 02:00










Mathematics, 22.06.2019 02:10

Mathematics, 22.06.2019 02:10

Mathematics, 22.06.2019 02:10

![\eta_{a} = [a]*Va = 0.110 M * 1.41 L = 0.155 moles \thinspace acid](/tpl/images/0566/2281/d601e.png)
![\eta_{b} = [b]*Vb = 0.253 M * 1.41 L = 0.357 moles\thinspace propanoate](/tpl/images/0566/2281/b75d2.png)