Chemistry, 24.03.2020 14:31 BurwinkelElla19
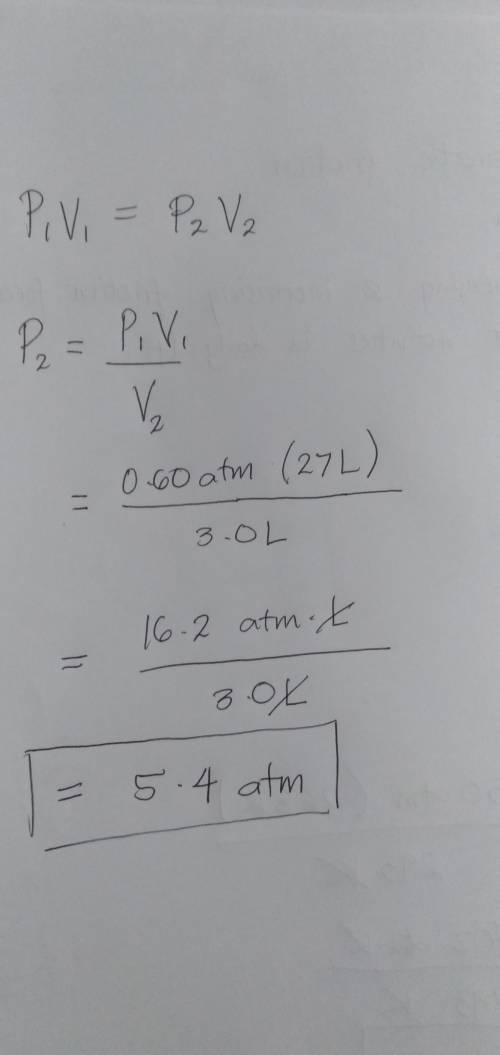
A gas under an initial pressure of 0.60 atm is compressed at constant temperature from 27 L to 3.0 L. The final pressure becomes .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1


Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

You know the right answer?
A gas under an initial pressure of 0.60 atm is compressed at constant temperature from 27 L to 3.0 L...
Questions


Chemistry, 25.03.2020 23:38

World Languages, 25.03.2020 23:38


Mathematics, 25.03.2020 23:38

Mathematics, 25.03.2020 23:38

Arts, 25.03.2020 23:38

Social Studies, 25.03.2020 23:38


English, 25.03.2020 23:38

Mathematics, 25.03.2020 23:38






Mathematics, 25.03.2020 23:38