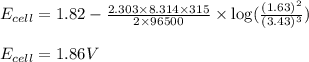A galvanic cell at a temperature of 42 degrees Celcius is powered by the following redox reaction:
3CU2+(aq) + 2Al(s) > 3Cu(s) + 2Al3+(aq)
Suppose the cell is prepared with 3.43 M Cu2+n one half-cell and 1.63 M Al3+in the other.
A) Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
A galvanic cell at a temperature of 42 degrees Celcius is powered by the following redox reaction:
Questions



Mathematics, 26.06.2020 15:01

Health, 26.06.2020 15:01





History, 26.06.2020 15:01


Mathematics, 26.06.2020 15:01

English, 26.06.2020 15:01








Computers and Technology, 26.06.2020 15:01

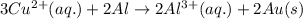
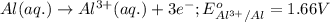 ( × 2)
( × 2)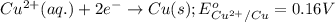 ( × 3)
( × 3)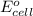 of the reaction, we use the equation:
of the reaction, we use the equation: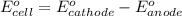
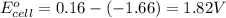
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Al^{3+}]^2}{[Cu^{2+}]^3}](/tpl/images/0557/5138/3aff8.png)
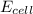 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![42^oC=[42+273]K=315K](/tpl/images/0557/5138/563a7.png)
![[Al^{3+}]=1.63M](/tpl/images/0557/5138/1e5e1.png)
![[Cu^{2+}]=3.43M](/tpl/images/0557/5138/455ea.png)