
Chemistry, 19.03.2020 17:10 studybuddy0203
Predict the effect of increasing the container volume on the amounts of each reactant and product in the following reactions: (a) CH3OH(l) <--> CH3OH(g) (b) CH4(g) + NH3(g) <--> HCN(g) + 3H2(g)

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1

Chemistry, 23.06.2019 08:30
Of the following elements, which is the least reactive? a. c b. h c. li d. he
Answers: 1
You know the right answer?
Predict the effect of increasing the container volume on the amounts of each reactant and product in...
Questions




Mathematics, 04.01.2020 18:31

History, 04.01.2020 18:31

Geography, 04.01.2020 18:31






Arts, 04.01.2020 18:31


Mathematics, 04.01.2020 18:31





Biology, 04.01.2020 18:31



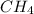 and 1 mol of
and 1 mol of 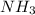 ) and in the right side we will have 4 moles (1 mol of
) and in the right side we will have 4 moles (1 mol of 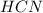 and 3 mol of
and 3 mol of 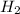 ). Therefore, the reaction will move to the right side.
). Therefore, the reaction will move to the right side.

