Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
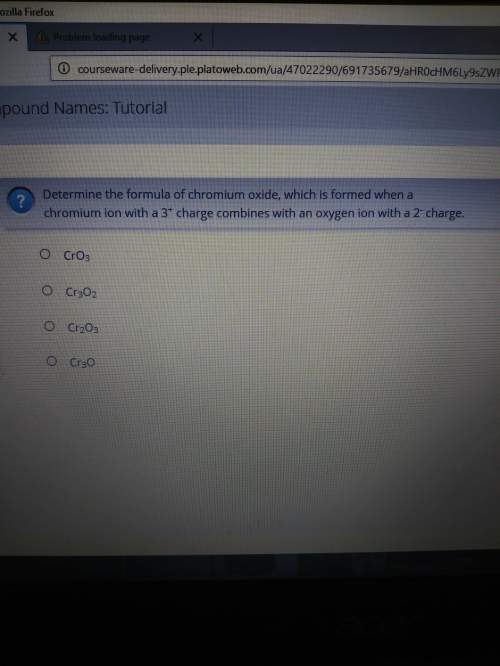
Determine the formula of chromium oxide, which is formed when a chromium ion with a 3+ charge combin...
Questions

Mathematics, 04.12.2020 09:50



Mathematics, 04.12.2020 09:50



History, 04.12.2020 09:50


Chemistry, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00


Mathematics, 04.12.2020 14:00


Mathematics, 04.12.2020 14:00

Chemistry, 04.12.2020 14:00

Chemistry, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00


Mathematics, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00