

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
For the reaction 2 H2S(g) D 2 H2 (g) + S2 (g), Kp = 1.5 × 10−5 at 800.0°C. If the initial partial pr...
Questions

Business, 09.04.2021 14:00

Mathematics, 09.04.2021 14:00

Mathematics, 09.04.2021 14:00



Physics, 09.04.2021 14:00




Engineering, 09.04.2021 14:00

Chemistry, 09.04.2021 14:00



English, 09.04.2021 14:00


English, 09.04.2021 14:00


Mathematics, 09.04.2021 14:00


Chemistry, 09.04.2021 14:00

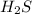 is 3.92 atm
is 3.92 atm 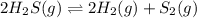
![K_p=\frac{[H_2]^2\times [S_2]}{[H_2S]^2}](/tpl/images/0553/9888/9721f.png)
![1.5\times 10^{-5}=\frac{[H_2]^2\times [S_2]}{[H_2S]^2}](/tpl/images/0553/9888/52be4.png)
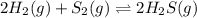
![K_p=\frac{[H_2S]^2}{[H_2]^2\times [S_2]}](/tpl/images/0553/9888/fc7bb.png)
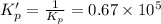
![0.67\times 10^5=\frac{2x]^2}{[4.00-2x]^2\times [2.00-x]}](/tpl/images/0553/9888/97489.png)
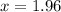
![[H_2S]=2x=2\times 1.96=3.92 atm](/tpl/images/0553/9888/8a012.png)


