

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

You know the right answer?
Use values of Ksp for AgI and Kf for Ag(CN)2− to calculate the molar solubility of AgI in pure water...
Questions

Business, 15.12.2020 09:20






English, 15.12.2020 09:20





Biology, 15.12.2020 09:20

Biology, 15.12.2020 09:20


English, 15.12.2020 09:20





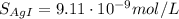
![S_{[ AgI]} = 0.05 M](/tpl/images/0550/2696/fce57.png)
![K_{sp} = [Ag^{+}][I^{-}] = 8.3\cdot 10^{-17}](/tpl/images/0550/2696/5785c.png)
![K_{sp} = [Ag^{+}]^{2} \rightarrow S = \sqrt{K_{sp}} = \sqrt{8.3\cdot 10^{-17}} = 9.11 \cdot 10^{-9} mol/L](/tpl/images/0550/2696/2ba74.png)
![K_{sp} = [Ag^{+}][I^{-}]](/tpl/images/0550/2696/d6ef6.png) (2)
(2)![K_{f} = \frac{[Ag(CN)_{2}^{-}]}{[Ag^{+}][CN^{-}]^{2}}](/tpl/images/0550/2696/5bfe2.png) (4)
(4)![K_{eq} = \frac{[Ag(CN)_{2}^{-}][I^{-}]}{[CN^{-}]^{2}}](/tpl/images/0550/2696/ca1d7.png) (6)
(6)![[I^{-}] = \frac{K_{sp}}{[Ag^{+}]}](/tpl/images/0550/2696/b9a73.png) (7)
(7)![K_{eq} = \frac{[Ag(CN)_{2}^{-}]}{[CN^{-}]^{2}}*\frac{K_{sp}}{[Ag^{+}]}](/tpl/images/0550/2696/45445.png)
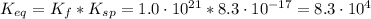
![K_{eq} = \frac{[Ag(CN)_{2}^{-}][I^{-}]}{[CN^{-}]^{2}} = \frac{x*x}{(0.1 - 2x)^{2}}](/tpl/images/0550/2696/83c45.png)
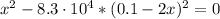
![x = 0.05 mol/L = S_{[ AgI]}](/tpl/images/0550/2696/daf71.png)


