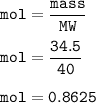Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
How many milliliters of sodium metal, with a density of 0.97 g/mL, would be needed to produce 34.5 g...
Questions

Biology, 03.08.2019 22:30

History, 03.08.2019 22:30

Mathematics, 03.08.2019 22:30



Biology, 03.08.2019 22:30

English, 03.08.2019 22:30

Mathematics, 03.08.2019 22:30

Mathematics, 03.08.2019 22:30

Mathematics, 03.08.2019 22:30


Business, 03.08.2019 22:30

History, 03.08.2019 22:30

Social Studies, 03.08.2019 22:30

Biology, 03.08.2019 22:30

Social Studies, 03.08.2019 22:30

Social Studies, 03.08.2019 22:30