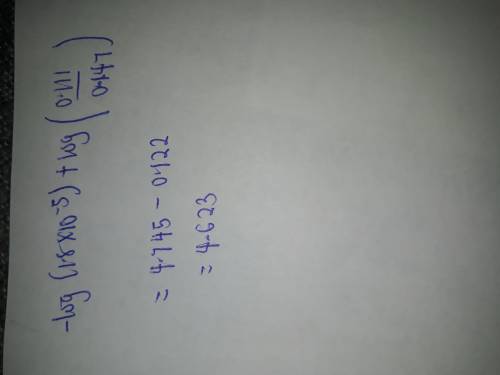Chemistry, 16.03.2020 21:31 ayoismeisalex
Calculate the pH after 0.018 mole of HCl is added to 1.00 L of each of the four solutions. (Assume that all solutions are at 25°C.) (a) 0.129 M acetic acid (HC2H3O2, Ka = 1.8 ✕ 10−5) (b) 0.129 M sodium acetate (NaC2H3O2) (c) pure H2O (d) 0.129 M HC2H3O2 and 0.129 M NaC2H3O2

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Calculate the pH after 0.018 mole of HCl is added to 1.00 L of each of the four solutions. (Assume t...
Questions

Computers and Technology, 16.08.2021 14:00

Business, 16.08.2021 14:00

Social Studies, 16.08.2021 14:00



Mathematics, 16.08.2021 14:00

Computers and Technology, 16.08.2021 14:00


Social Studies, 16.08.2021 14:00

Physics, 16.08.2021 14:00

Mathematics, 16.08.2021 14:00

Mathematics, 16.08.2021 14:00

Mathematics, 16.08.2021 14:00


Business, 16.08.2021 14:00

Business, 16.08.2021 14:00