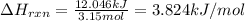3.15 mol of an unknown solid is placed into enough water to make 150.0 mL of solution. The solution's temperature increases by 16.01°C. Calculate ∆H for the dissolution of the unknown solid. (The specific heat of the solution is 4.18 J/g・°C and the density of the solution is 1.20 g/mL).

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
3.15 mol of an unknown solid is placed into enough water to make 150.0 mL of solution. The solution'...
Questions

History, 03.03.2021 14:00


History, 03.03.2021 14:00

Mathematics, 03.03.2021 14:00



English, 03.03.2021 14:00

Biology, 03.03.2021 14:00


Mathematics, 03.03.2021 14:00

Mathematics, 03.03.2021 14:00

Mathematics, 03.03.2021 14:00

English, 03.03.2021 14:00



Biology, 03.03.2021 14:00

Mathematics, 03.03.2021 14:00

Chemistry, 03.03.2021 14:00


Social Studies, 03.03.2021 14:00

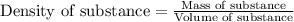
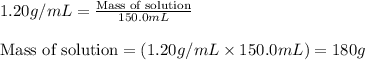
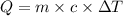
 = change in temperature = 16.01°C
= change in temperature = 16.01°C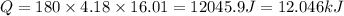
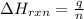
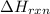 = enthalpy change of the reaction
= enthalpy change of the reaction