Chemistry, 16.03.2020 20:30 Tyrant4life
Many portable gas heaters and grills use propane, C3H8(g). Using enthalpies of formation, calculate the quantity of heat produced when 13.0 g of propane is completely combusted in air under standard conditions. Assume that liquid water is forming. Express the heat in kilojoules to three significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 11:30
The dashed segment of the plotted experiment in the graph in the l
Answers: 3

Chemistry, 23.06.2019 15:30
Dona wrote the characteristics of two types of galaxies as shown below: type a: has a large flattened core type b: does not have a regular shape which statement is correct? type a is an irregular galaxy and type b is a lens galaxy. type a is a lens galaxy and type b is an irregular galaxy. type a is a spiral galaxy and type b is an elliptical galaxy. type a is an elliptical galaxy and type b is a spiral galaxy.
Answers: 2
You know the right answer?
Many portable gas heaters and grills use propane, C3H8(g). Using enthalpies of formation, calculate...
Questions



English, 01.02.2021 03:10

Mathematics, 01.02.2021 03:10

Mathematics, 01.02.2021 03:10



Mathematics, 01.02.2021 03:10

History, 01.02.2021 03:10


Mathematics, 01.02.2021 03:10




Mathematics, 01.02.2021 03:10

History, 01.02.2021 03:10

Biology, 01.02.2021 03:10

Mathematics, 01.02.2021 03:10


![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0549/0754/e893d.png)
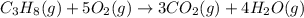
![\Delta H_{rxn}=[(3\times \Delta H_f_{(CO_2(g))})+(4\times \Delta H_f_{(H_2O(g))})]-[(1\times \Delta H_f_{(C_3H_8(g))})+(5\times \Delta H_f_{(O_2(g))})]](/tpl/images/0549/0754/b4bd0.png)
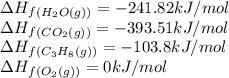
![\Delta H_{rxn}=[(3\times (-393.51))+(4\times (-241.82))]-[(1\times (-103.8))+(3\times (0))]\\\\\Delta H_{rxn}=-2044.01kJ/mol](/tpl/images/0549/0754/08c86.png)
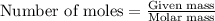
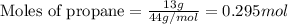
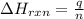
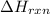 = enthalpy change of the reaction = -2044.01 kJ/mol
= enthalpy change of the reaction = -2044.01 kJ/mol