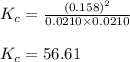Chemistry, 16.03.2020 16:29 jennemylesp19oy5
Suppose that 0.1000 mole each of H2and I2are placed in a 1.000-L flask, stoppered, and the mixture is heated to 425oC. At equilibrium, the concentration of I2is found to be 0.0210 M. Calculate Kcfor the following reaction at 425oC. H2(g) + I2(g) ⇄2 HI(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:50
What type of reaction is illustrated? 2c12o5 = 2cl2 + 502
Answers: 2


Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Suppose that 0.1000 mole each of H2and I2are placed in a 1.000-L flask, stoppered, and the mixture i...
Questions










Mathematics, 12.03.2020 00:00



Advanced Placement (AP), 12.03.2020 00:00







Mathematics, 12.03.2020 00:01

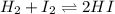
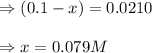
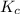 for above equation follows:
for above equation follows:![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0548/4996/62646.png)
![[HI]_{eq}=2x=(2\times 0.079)=0.158M](/tpl/images/0548/4996/cad3c.png)
![[H_2]_{eq}=(0.1-x)=(0.1-0.079)=0.0210M](/tpl/images/0548/4996/029c8.png)
![[I_2]_{eq}=0.0210M](/tpl/images/0548/4996/0be20.png)