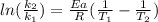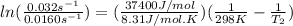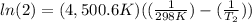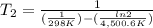Chemistry, 12.03.2020 20:03 shelbylynn17
The activation energy of a certain reaction is 37.4 kJ/mol . At 25 ∘C , the rate constant is 0.0160s−1 . At what temperature in degrees Celsius would this reaction go twice as fast?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2


Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
The activation energy of a certain reaction is 37.4 kJ/mol . At 25 ∘C , the rate constant is 0.0160s...
Questions

Mathematics, 06.01.2021 01:00


Mathematics, 06.01.2021 01:00

Mathematics, 06.01.2021 01:00

Mathematics, 06.01.2021 01:00

English, 06.01.2021 01:00

Biology, 06.01.2021 01:00



Mathematics, 06.01.2021 01:00


History, 06.01.2021 01:00


English, 06.01.2021 01:00



Mathematics, 06.01.2021 01:00


Mathematics, 06.01.2021 01:00

Chemistry, 06.01.2021 01:00