Chemistry, 12.03.2020 00:41 ayoismeisalex
A solution of hydrochloric acid of unknown concentration was titrated with 0.10 M NaOH. If a 100.-mL sample of the HCl solution required exactly 1.0 mL of the NaOH solution to reach the equivalence point, what was the initial pH of the HCl solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2
You know the right answer?
A solution of hydrochloric acid of unknown concentration was titrated with 0.10 M NaOH. If a 100.-mL...
Questions

Mathematics, 28.09.2020 14:01

History, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01














Mathematics, 28.09.2020 14:01



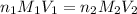
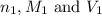 are the n-factor, molarity and volume of acid which is HCl
are the n-factor, molarity and volume of acid which is HCl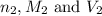 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.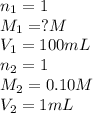
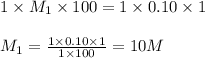
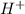 ions and 1 mole of
ions and 1 mole of 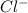 ions
ions![pH=-\log[H^+]](/tpl/images/0543/9407/cf945.png)
![[H^+]=0.001M](/tpl/images/0543/9407/58906.png)