
At a certain temperature this reaction follows second-order kinetics with a rate constant of Suppose a vessel contains at a concentration of . Calculate the concentration of in the vessel seconds later. You may assume no other reaction is important. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2



Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
At a certain temperature this reaction follows second-order kinetics with a rate constant of Suppose...
Questions


Biology, 16.03.2020 18:48








Computers and Technology, 16.03.2020 18:49







Health, 16.03.2020 18:49


History, 16.03.2020 18:49

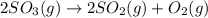
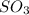 in the vessel after 0.240 seconds is 0.24 M
in the vessel after 0.240 seconds is 0.24 M![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0541/9921/5ea71.png)
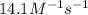
![[A]_o](/tpl/images/0541/9921/9caf5.png) = Initial concentration = 1.44 M
= Initial concentration = 1.44 M![14.1=\frac{1}{0.240}\left (\frac{1}{[A]}-\frac{1}{1.44}\right)](/tpl/images/0541/9921/8dfd8.png)
![[A]=0.245M](/tpl/images/0541/9921/0a4f3.png)


