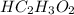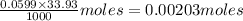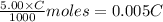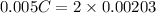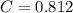Chemistry, 10.03.2020 05:58 ayoismeisalex
A 5.00 mL sample of vinegar, an aqueous solution of acetic acid (HC2H3O2), is titrated with 0.0599 M Ca(OH)2, and 33.93 mL of the Ca(OH)2 solution is required to reach the equivalence point. What is the molarity of the acetic acid? Group of answer choices

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
A 5.00 mL sample of vinegar, an aqueous solution of acetic acid (HC2H3O2), is titrated with 0.0599 M...
Questions

Mathematics, 26.03.2020 15:59


Mathematics, 26.03.2020 15:59

Mathematics, 26.03.2020 16:00



English, 26.03.2020 16:12

History, 26.03.2020 16:12

Mathematics, 26.03.2020 16:12

Mathematics, 26.03.2020 16:12



English, 26.03.2020 16:13




Mathematics, 26.03.2020 16:13



History, 26.03.2020 16:13

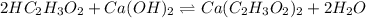
 neutralizes 2 moles of
neutralizes 2 moles of