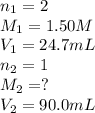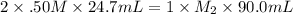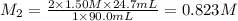Chemistry, 10.03.2020 04:01 marygatewell385
A volume of 90.0 mLmL of aqueous potassium hydroxide (KOHKOH) was titrated against a standard solution of sulfuric acid (H2SO4H2SO4). What was the molarity of the KOHKOH solution if 24.7 mLmL of 1.50 MM H2SO4H2SO4 was needed

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
A volume of 90.0 mLmL of aqueous potassium hydroxide (KOHKOH) was titrated against a standard soluti...
Questions




English, 04.11.2020 06:30

English, 04.11.2020 06:30

History, 04.11.2020 06:30

Chemistry, 04.11.2020 06:30



History, 04.11.2020 06:30


Mathematics, 04.11.2020 06:30


Mathematics, 04.11.2020 06:30

Chemistry, 04.11.2020 06:30


Mathematics, 04.11.2020 06:30


Mathematics, 04.11.2020 06:30

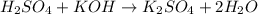 (Neutralization reaction)
(Neutralization reaction)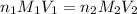
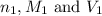 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 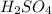
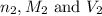 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.