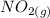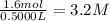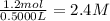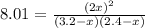Chemistry, 10.03.2020 00:27 TabbyKun00
Suppose a 500.mL flask is filled with 1.6mol of NO3 and 1.2mol of NO2 . The following reaction becomes possible: +NO3gNOg 2NO2g The equilibrium constant K for this reaction is 8.01 at the temperature of the flask. Calculate the equilibrium molarity of NO3 . Round your answer to two decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
Suppose a 500.mL flask is filled with 1.6mol of NO3 and 1.2mol of NO2 . The following reaction becom...
Questions


English, 15.03.2020 07:00

Health, 15.03.2020 07:01




Mathematics, 15.03.2020 07:02



Social Studies, 15.03.2020 07:02







History, 15.03.2020 07:04


Mathematics, 15.03.2020 07:05

Social Studies, 15.03.2020 07:08

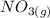 is 1.60 M
is 1.60 M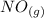 ⇄2
⇄2